Ongoing Research
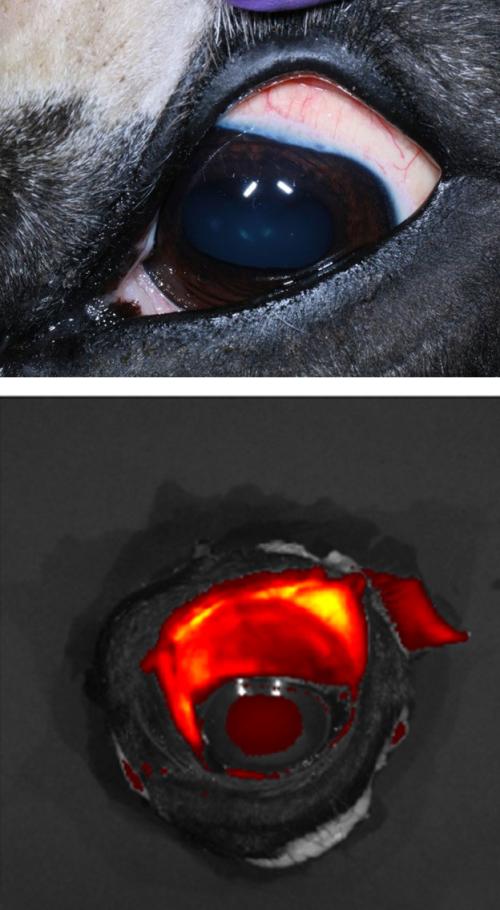
Subconjunctival Poly I:C MSC Therapy
Dr. Cassano’s laboratory is concluding a trial that is investigating the safe use and migration of subconjunctival injected toll-like receptor ligand poly I:C-activated mesenchymal stem cell therapy as an effective treatment method for equine recurrent uveitis (ERU). Previous research demonstrated the immunomodulatory properties of poly I:C-activated (pIC) mesenchymal stem cells (MSCs) injected in the subconjunctiva of the horse resulting in significantly reduced intra-ocular pressure with no side-effects following injection. The aims of the study are to evaluate the duration the MSCs remain at the injection site using fluorescently-labelled MSCs, and to note the effects of pIC-activated MSCs on the periocular environment. Pending final ocular histopathology assessment, this method of stem cell treatment would be valid as a double-blinded clinical trial for ERU as well as other ocular conditions.
MSC Antimicrobial Peptide Secretion
The Cassano Lab has partnered with The Gelli Lab at UC Davis Genome and Biomedical Sciences facility to further investigate the antimicrobial effects of MSCs. Working from the research published by Rebecca Harman et. al which demonstrated that the secretome of equine MSCs contain multiple anti-microbial peptides (AMPs), factors that disrupt the membrane integrity of bacteria commonly found in skin wounds, cysteine proteases that decrease the viability of mature methicillin-resistant Staphylococcus aureus bacteria biofilms, and CCL2 which acted to stimulate the production of antimicrobial peptides in equine keratinocytes. Dr. Cassano’s study aims to further investigate the antimicrobial properties of equine MSCs using Staphylococcus aureus, Candida albicans, and Cryptococcus neoformans, and see if a co-culture with any of these pathogens would act to suppress pathogen growth. Further investigations will look into the use of canine and feline MSCs and if the use of MSCs could prove to be a viable clinical treatment for infections of these pathogens.
MSCs on Synovitis
The Cassano Lab has partnered with the laboratory of Dr. Thomas Koch for a clinical trial to investigate the use of allogeneic equine cord-blood MSCs as a treatment for synovitis in client-owned horses. This clinical trial aims to investigate mesenchymal stromal cell therapy to help decrease inflammation and regenerate damaged tissues associated with synovitis.
Mitochondria (MT) play a crucial role in the maintenance of cellular homeostasis. MT injury is deleterious and is involved in the pathogenesis of multiple disorders including osteoarthritis (OA). MT dysfunction amplifies the response to cytokine-induced chondrocyte inflammation. Mitotherapy, as a therapeutic approach for synovitis and OA, is comprehensive and focused on cell metabolism rather than focused on a single signaling pathway. We postulate that MT replacement for augmentation of MT damage may become a novel and effective therapeutic intervention. In cells, MT dynamics is orchestrated by the processes of fusion, fission and mitophagy. We believe that exogenous MT will “repair” recipient cells residing in the joint cavity through fusion with host MT. MT fusion results in the exchange of metabolites, proteins, peptides and mtDNA with resultant improvement of MT condition within cell. Fusion between impaired and healthy MT restored proper functionality to damage organelles.
We hypothesize that viable MT isolated from the horse’s own blood and delivered to the joint cavity by intra-articular injection will augment and replace the damaged MT in recipient chondrocytes and synovial cells and deliver bioactive molecules including antioxidants. Oxidative stress and inflammatory processes are interdependent. Recently, MT, derived from a number of different tissue sources, have been shown to alter the metabolic profile of injured/inflamed cells and induce regenerative processes. The administration of exogenous MT into the heart in a pig model of ischemic myopathy decreased TNFα, IL-6, IL-10, and MCP-1 levels. MSCs also work, in part, through MT transfer when they transfer their own MT to replace defective mitochondria or compensate for mitochondrial malfunction of damaged inflamed cells. This transfer of MT between MSCs and damaged tissues is a key mechanism for tissue repair/regeneration. MT transfer directs polarization of macrophages towards M2 macrophages via enhancement of oxidative phosphorylation, which leads to the production of anti-inflammatory cytokines, secretion of extracellular matrix proteins and secretion of pro-regenerative factors.
Our central hypothesis is that intra-articular MT injection in horses with synovitis is an ideal treatment for reduction of joint inflammation and induction of pro-regenerative protective mechanisms and may become an innovative strategy for treating synovitis and preventing OA. Therefore, we aim to perform intra-articular injection of autologous, peripheral blood mononuclear cell (PBMC) derived MT in order to ameliorate joint inflammation.
